(Chemistry) ICSE Class X Important Questions : Chemistry (1996)
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in
Paper : ICSE Class X Important Questions : Chemistry (1996)
General Instructins
- Section I is compulsory. Attempt any four questions from Section II.
- The intended marks for questions or parts of questions are given in brackets.
SECTION I (40 Marks)
Attempt all questions from this Section
Question 1
(a) From the following list of substances, choose the substances which meet the
description given in parts (i) to (v) below:
Ammonium chloride, ammonium nitrate, chlorine, dilute hydrochloric acid, iron,
lead nitrate, manganese (IV) oxide, silver nitrate, sodium nitrate, sodium
nitrite, sulphur. [6]
- Two compounds heated together in solution to produce nitrogen.
- An element which exists in two crystalline forms.
- A compound which on heating gives oxygen as the only gaseous product.
- A substance containing both molecules and ions.
- Two compounds whose aqueous solution give white precipitates with dilute hydrochloric acid.
(b) What do you see when: [6]
- Sodium hydroxide solution is added to zinc sulphate solution till it is in excess.
- Chlorine water is exposed to sunlight.
- Ammonia gas is bubbled through red litmus solution.
- Barium chloride solution is added to dilute sulphuric acid.
(c) Explain why the following statements are not correct: [6]
- The element nitrogen can be obtained in the pure state by removing carbon dioxide and oxygen from air.
- Ammonium salts will, on heating, decompose to give ammonia.
- Lead chloride can be prepared by adding dilute hydrochloric acid to lead sulphate solution
- 1 gram of any gas occupies 22.4 litres at S.T.P.
(d) [4]
- A solution has a pH of 7. Explain how you would:
1. Increase its pH; 2. Decrease its pH. - If a solution changes the colour of litmus from red to blue, what can you say about its pH?
- What can you say about the pH of a solution that liberates carbon dioxide from sodium carbonate?
(e) [8]
- Under the same conditions of temperature and pressure you collect 2 litres
of carbon dioxide, 3 litres of chlorine, 5 litres of hydrogen, 4 litres of
nitrogen and 1 litre of sulphur dioxide. In which gas sample will there be:
1. The greatest number of molecules;
2. The least number of molecules? Just your answer. - The pressure on one mole of gas at S.T.P. is doubled and the temperature is raised to 546 K. What is the final volume of the gas?
- Find the total percentage of oxygen in magnesium nitrate crystals: Mg(NO3)2.6H2O.
(H = 1 , N = 14 , O = 16 , Mg = 24)
(f) You are given the three white powders calcium carbonate, lead carbonate and zinc carbonate. Describe the tests you would carry out in solution to identify the metal in each of the above compounds. Indicate clearly how you would prepare the solutions for the tests. [5]
(g) Write the equation for each of the following reactions: [5]
- Action of heat on potassium nitrate.
- Chlorine is passed over heated iron.
- Chlorine is passed into pure water.
- Solutions of ammonium chloride and sodium hydroxide are mixed and heated.
- Copper sulphate solution is added to sodium hydroxide solution.
SECTION II (40 marks)
Answer any four questions
Question 2
(a) [7]
- What is the purpose of the Haber Process?
- Name the gaseous inputs of the Haber Process and state the ratio by volume in which the gases are mixed.
- What is done to increase the rate of the reaction in the Haber process?
- Give two different ways by which the product can be separated from the reactants.
(b) A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen. Determine the empirical formula of this compound.[3]
Question 3
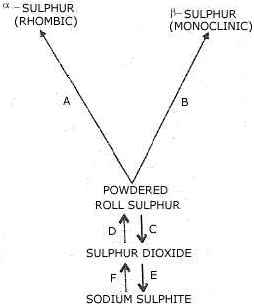
The following questions refer to the scheme above.
(a) Describe briefly how you would carry out each of the changes A and B. [6]
(b) Write one equation in each case for a reaction which could bring about each of the changes C, D (Sulphur dioxide to sulphur), E and F. [4]
Question 4 [5]
(a) Find the relative molecular mass of a gas, 0.546g of which occupies 360 cm3 at 87°C at 380 mm Hg pressure.
(b) [5]
- What volume of hydrogen sulphide at STP will burn in oxygen to yield 12.8
g sulphur dioxide according to the equation:
2H2S + 3O2 ———> 2H2O + 2SO2
(H = 1, O = 16, S = 32) - For the volume of hydrogen sulphide determined in (b) (i) above, what volume of oxygen would be required for complete combustion?
Question 5
(a) For each substance listed below, explain its significance in the extraction
of aluminium: [4]
- Bauxite
- Sodium hydroxide
- Cryolite
- Graphite
(b) The following questions related to the extraction of aluminium by electrolysis: [3]
- Give the equation for the reaction that takes place at the cathode.
- Explain why it is necessary to renew the anode from time to time.
(c) [3]
- What is an alloy?
- An alloy usually has some property which makes it particularly useful.
What is the special property of:
1. Duralumin 2. Type metal
Question 6
(a) Name from the list of substances given below, the substances which you would
use to prepare each of the following salts, named in parts from (i) to (iv):
The substances are:
(Copper, Lead, Sodium, Zinc, Copper oxide, Lead carbonate, Sodium carbonate
solution, Dilute hydrochloric acid, Dilute nitric acid and Dilute sulphuric
acid): [5]
- Zinc sulphate;
- Copper sulphate;
- Sodium sulphate;
- Lead sulphate.
(b) Sulphur dioxide and chlorine are both used as bleaching agents: [5]
- What is similar in the use of chlorine and sulphur dioxide as bleaching agents?
- How does the bleaching action of these two gases differ?
- What type of fibre should not be bleached using chlorine? Why should the use of chlorine be avoided for this fibre?
Question 7
(a) Give one example in each case of a substance which contains: [3]
- Ions only;
- Molecules only;
- Both ions and molecules.
(b) [3]
- What is meant by the term ‘electrolyte’?
- What are the particles present in a compound which is a non-electrolyte?
- If an electrolyte is described as a ‘strong electrolyte’, what does this mean?
(c) The following questions refer to the electrolysis of copper sulphate solution with copper electrodes: [4]
- Compare the change in mass of the cathode with the change in mass of the anode.
- What is seen to happen to the colour of the copper sulphate solution if platinum electrodes are used? Explain this observation.
- What is the practical application of the electrolysis of copper sulphate solution? Briefly describe one such application.