(Chemistry) ICSE Class X Important Questions : Chemistry (2004)
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in
Paper : ICSE Class X Important Questions : Chemistry (2004)
General Instructions
- Section I is compulsory. Attempt any four questions from Section II.
- The intended marks for questions or parts of questions are given in brackets [ ].
SECTION I (40 Marks)
Attempt all questions from this Section
Question 1
(a) Choose the letters A, B, C or D to match the descriptions from (i) to (vi)
given below: [6]
A. Ammonia
B. Hydrogen Chloride
C. Hydrogen sulphide
D. Sulphur dioxide.
- This gas can be oxidized to sulphur.
- This gas decolourises potassium permanganate solution.
- When this gas is bubbled through copper sulphate solution, a deep blue coloured solution is formed.
- This gas gives a white precipitate when reacted with silver nitrate solution acidified with dilute nitric acid.
- This gas burns in oxygen with a green flame.
- This gas can be obtained by the reaction between copper and concentrated sulphuric acid.
(b) When heated, potassium permanganate decomposes according to the following equation
2KMnO4→K2MnO4+ MnO2 + O2
Solid residue
(i) Some potassium permanganate was heated in a test tube. After collecting one litre of oxygen at room temperature it was found that the test-tube had undergone a loss in mass of 1.32 g. If one litre of hydrogen under the same conditions of temperature and pressure has a mass of 0.0825 g, calculate the relative molecular mass of oxygen.
(ii) Given that the molecular mass of potassium permanganate is 158, what volume of oxygen (measured at room temperature) would be obtained by the complete decomposition of 15.8 g of potassium permanganate? (Molar volume at room temperature is 24 litres.)
(c) X, Y and Z are three crystalline solids which are soluble in water and have a common anion. To help you to identify X, Y and Z you are provided with the following experimental observations. Copy and complete the corresponding inferences in (i) to (iv). [6]
- A reddish-brown gas is obtained when X, Y and Z are separately warmed with
concentrated sulphuric acid and copper turnings added to the mixture.
INFERENCE 1: The common anion is the ........ ion. - When X is heated, it melts and gives off only one gas which re-lights a
glowing splint.
INFERENCE 2: The cation in X is either ....... or ...... - The action of heat on Y produces a reddish-brown gas and a yellow residue
which fuses with the glass of the test-tube.
INFERENCE 3: The metal ion present in Y is the ......... ion.
- When Z is heated it leaves no residue. Warming Z with sodium hydroxide
solution liberates a gas which turns moist red litmus paper blue.
INFERENCE 4: Z contains the .......... cation.
- Write the equations for the following reactions:
1. X and concentrated sulphuric acid (below 2000C).
(One equation only for either of the cations given in INFERENCE 2.)
2. Action of heat on Y.
(d) The electronegativities (according to Pauling) of the elements in period 3 of the Periodic Table are as follows with the elements arranged in alphabetical order: [6]
| Al | Cl | Mg | Na | P; | S | Si |
| 1.5 | 3.0 | 1.2 | 0.9 | 2.1 | 2.5 | 1.8 |
(i) Arrange the elements in the order in which they occur in the Periodic Table from left to right. (The group 1 element first, followed by the group 2 element and so on, upto group 7.)
(iii) Choose the word or phrase from the brackets which correctly completes each of the following statements.
- The element below sodium in the same group would be expected to have a ............. (lower/higher) electronegativity than sodium and the Y element above chlorine would be expected to have a ............... (lower/higher) ionization potential than chlorine.
- On moving from left to right in a given period, the number of shells (remains the same/increases/decreases).
- On moving down a group, the number of valence electrons (remains the same/increases/decreases).
(e) Write balanced equations for the following reactions: [6]
- Chlorine is passed into an aqueous solution of sulphur dioxide.
- Aluminium powder is warmed with hot and concentrated caustic soda solution.
- Concentrated nitric acid is added to copper turnings kept in a beaker.
- Red lead (trilead tetroxide) is warmed with concentrated hydrochloric acid.
- Chlorine gas is passed through an aqueous solution of iron (II) sulphate acidified with dilute sulphuric acid.
- Ethane is burnt in air.
(f) Sodium hydroxide solution is added first in a small quantity, then in excess to the aqueous salt solutions of copper (II) sulphate, zinc nitrate, lead nitrate, calcium chloride and iron (III) sulphate. Copy the following table and write the colour of the precipitate in (i) to (v) and the nature of the precipitate (soluble or insoluble) in (vi) to (x). [5]
| Aqueous salt solution | Colour of precipitate when NaOH is added in a small quantity | Nature of precipitate (soluble or insoluble) when NaOH is added in excess. |
| Copper (II) sulphate | (i) | (vi) |
| Zinc nitrate | (ii) | (vii) |
| Lead nitrate | (iii) | (viii) |
| Calcium chloride | (iv) | (ix) |
| Iron (III) sulphate | (v) | (x) |
(g) Which of the following methods A, B, C, D or E is generally used for preparing the chlorides listed below from (i) to (v). Answer by writing down the chloride and the letter pertaining to the corresponding method. Each letter is to be used only once. [5]
A. Action of an acid on a metal
B. Action of an acid on an oxide or carbonate
C. Direct combination
D. Neutralization of an alkali by an acid
E. Precipitation (double decomposition)
(i) Copper (II) chloride
(ii) Iron (II) chloride
(iii) Iron (III) chloride
(iv) Lead (II) chloride
(v) Sodium chloride
SECTION II (40 marks)
Answer any four questions from this section
Question 2
(a) Element X is a metal with a valency 2. Element Y is a non-metal with a
valency 3. [5]
- Write equations to show how X and Y form ions.
- If Y is a diatomic gas, write the equation for the direct combination of X and Y to form a compound.
- Write two applications of electrolysis in which the anode diminishes in mass.
- If the compound formed between X and Y is melted and an electric current passed through the molten compound, the element X will be obtained at the ........... and Y at the ........... of the electrolytic cell. (Provide the missing words.)
(b) [5]
- What kind of particles will be found in a liquid compound which is a non-electrolyte?
- If HX is a weak acid, what particles will be present in its dilute solution apart from those of water?
- (iii) Cations are formed by .......... (loss/gain) of electrons and anions are formed by .......... (loss/gain) of electrons. (Choose the correct words to fill in the blanks)
- (iv) What ions must be present in a solution used for electroplating a particular metal?
- (v) Explain how electrolysis is an example of redox-reaction?
Question 3
(a) A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution:
| S . No. | Substances Added | Gas evolved | Odour |
| 1. | Calcium carbonate | ||
| 2. | Magnesium ribbon | ||
| 3. | Manganese (IV) oxide with heating |
||
| 4. | Sodium sulphide |
Complete the table by writing the gas evolved in each case and its odour.
(b) Write equations for: [3]
- The action of hot, concentrated sodium hydroxide on chlorine.
- The reaction of chlorine with excess of ammonia.
- The action of chlorine with slaked lime.
(c) An experiment showed that in a lead chloride solution, 6.21 g of lead combined with 4.26 g of chlorine. What is the empirical formula of this chloride? (Pb = 207; Cl = 35.5) [3]
Question 4
(a) (i) Name the ore of zinc containing its sulphide.
(ii) In the process of extracting zinc, the above named ore is roasted. Write the equation for the reaction which takes place when the sulphide ore is roasted.
(iii) Name the substance used to reduce the roasted ore. Write the equation for the reaction.
(iv) 'Iron is removed from a blast furnace as a liquid'. State how zinc leaves a furnace.[5]
(b) Write the equations for the reaction of zinc with each of the following:
- Sodium hydroxide solution.
- Dilute sulphuric acid.
- Copper sulphate solution. [3]
(c) (i) To protect iron from rusting, it is coated with a thin layer of zinc. Name the process.
(ii) Name a non-metal that has a metallic lustre and sublimes on heating.[2]
Question 5
(a) A flask contain 3.2g of sulphur dioxide. Calculate the following:
- The moles of sulphur dioxide present in the flask.
- The number of molecules of sulphur dioxide present in the flask.
- The volume occupied by 3.2g of sulphur dioxide at S.T.P.(S = 32, 0 = 16)
(b) The reaction of potassium permanganate (VII) with
acidified iron (II) sulphate is given below:
2KMnO4+ 10FeSO4 + 8H2SO4 → K2SO4+
2MnSO4 + 5Fe2 (SO4)3 + 8H2O
If 15.8g of potassium permanganate (VII) was used in the reaction, calculate the
mass of iron (II) sulphate used in the above reaction.
(K = 39, Mn = 55, Fe = 56, S = 32, 0 = 16) [2]
(c) Fill in the blanks with suitable words.
Prismatic and plastic sulphur are two .......... of sulphur. Both are insoluble
in water. Prismatic sulphur is soluble in a volatile liquid named ........ while
plastic sulphur is insoluble in it. Prismatic sulphur is ........ in structure
whereas plastic sulphur is .......... [2]
Question 6
(a) Aluminium is extracted from its chief ore, Bauxite. The ore is first purified and the metal is extracted from it by electrolytic reduction.
- Write three balanced equations for the purification of bauxite by Hall's process.
- Name a chemical used for dissolving aluminium oxide. In which state of sub-division is the chemical used?
- Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
- Mention one reason for the use of aluminium in thermite welding. [6]
(b) (i)Write the equation for the reaction in the Haber process that forms ammonia.
(ii)&State the purpose of liquefying the ammonia produced in the process.
(iii) Predict the group of an element X if its atomic number is 16.
(iv) State one reason why tap water is not used to prepare a solution of silver nitrate in the laboratory.
Question 7
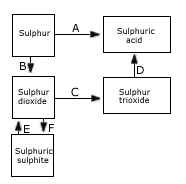
(a) (i) Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
(ii) In the Contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid
by reacting it with water. Instead a two step procedure is used. Write the equations for the two steps involved in D.
(iii) What type of substance will liberate sulphur dioxide from sodium sulphite in step E?
(iv) Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F. [5]
(b) (i) Write the equation for the preparation of ethylene from ethyl alcohol.
(ii) Write the general formula for a saturated hydrocarbon and give one example of a saturated hydrocarbon with its structural formula.
(iii) Name a compound which will give acetylene gas when
treated with water.
(iv) Which particular property of cast iron makes it unsuitable for the
construction of bridges? [5]