(Important Topics) Chemistry: Froth Floatation Process
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in

Important Topics Chemistry: Froth Floatation Process
In some cases for example, sulphides ores of copper, zinc and lead concentration is brought by this method. In this method advantage is taken of the referential wetting of the ore by an oil.The finely ground ore is taken in a tank containing water and 1% of pine oil or terpentine oil.
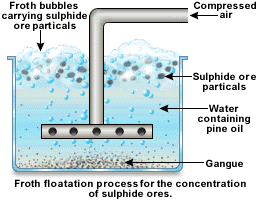
A strong current of air is blown through the suspension, producing a heavy froth or foam on the surface.The metal sulphide is wetted by the oil but the gangues is not and the sulphide-oil mixture is carried to the surface by films of oil The froth is skimmed off, the gangue settles down on the bottom or remains underneath the froth.By this floatation method it is possible to concentrate over 90% of a sulphite ore to 1/10 of its original bulk.
During the floatation process of some ores, these substances are added which activate or depress the floatation property of the minerals and thus help in the separation of minerals present in the ore. For instance, gaiena (PbS) is usually associated with sphalerite (ZnS) and pyrites (FeS2).
Concentration of galena is carried out by passing potassium ethyl xanthate (Collector) along with sodium cynamide and alkali (depressants) where by the floatation property of ZnS and FeS2 is depressed. Mainly PbS passes into the froth when air current in flown in, which is collected. After PbS is removed with the froth, same CuSO4 (activator) is added and air is blown.The floatation property of ZnS is increased which is now removed with the froth. The slurry is acidified and process is repeated when FeS2 passed into the froth and is collected.