(Important Topics) Chemistry: Rutherford’s Nuclear Model of Atom
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in

Important Topics Chemistry: Rutherford’s Nuclear Model of Atom
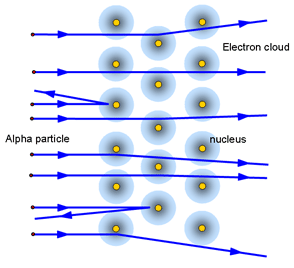
1) Rutherford carried out experiment on the bombardment of thin (10-4 mm) Au foil with high speed positively charged a- particles emitted from Ra and gave the following observations based on this experiment,
(i) Most of the a- particles passed without any
deflection.
(ii) Some of them were deflected away from their path.
(iii) Only a few (one in about 10,000) were returned back to their
original direction of propagation.
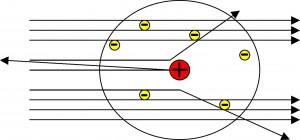
2) From the above observations he concluded that, an atom consists of :
(i) Nucleus which is small in size but carries the
entire mass i.e. contains all the neutrons and protons.
(ii) Extra nuclear part which contains This model was
similar to the solar system.
3) Properties of the nucleus
(i) Nucleus is a small, heavy, positively charged portion of the atom
and located at the centre of the atom.
(ii) All the positive charge of atom (i.e. protons) are present in
nucleus.
(iii) Nucleus contains neutrons and protons, and hence these particles
collectively are also referred to as nucleons.
(iv) The size of nucleus is measured in
Fermi (1 Fermi = 10-13 cm).
(v) The radius of nucleus is of the order of
1.5×10-13 cm to 6.5×10-13 cm i.e 1.5 to 6.5 fermi Generally the radius of the
nucleus (rn) is given by the following relation,
rn = ro(= 1.4 x 10-13 cm) x A1/3
This exhibited that nucleus is 10-5 times small in size as compared to the total size of atom.
(vi) The Volume of the nucleus is
about 1 and that of atom is 10-39cm3, i.e., volume of the
r is 10-5 times that of an atom.
(vii) The density of the nucleus is of the 10-15gcm3
or 108 tonnes cm-3 or 1012kg/cc.If nucleus is
spherical then,

4) Drawbacks of Rutherford’s model
(i) It does not obey the Maxwell theory of electrodynamics, according to it
“A small charged particle moving around an oppositely charged centre continously
loses its energy”. If an electron does so, it should continuously lose its
energy and should set up spiral motion ultimately failing into thenucleus.
(ii) It could not explain the line spectra of H- and
discontinuous spectrum nature.