TALLENTEX Papers 2021 - Class 10th
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in
TALLENTEX Papers 2021 - Class 9th
Section - A : Physics
Obejctive
1. The structure in the human ear that is responsible for converting sound energy into electri- cal energy is the:
(1) auditory nerve
(2) eardrum
(3) cochlea
(4) middle ear
2. What will be the resistance of a lamp, rated for 220V-500W and connected across a 110 volt supply ?
(1) 96.8 :
(2) 0.44 :
(3) 24.2 :
(4) 0.22 :
3. A ball rolls towards a wall along the floor, rebounds from the wall, and comes back to its original position. Which of the following statements is/are correct about the motion?
(i) Distance covered by the ball is 0.
(ii) Displacement of the ball is 0.
(iii)Average velocity of the ball is 0.
(1) Only (i)
(2) (i) and (iii)
(3) Only (ii)
(4) (ii) and (iii)
4. In the figure given below, the position–time graph of a particle of mass 0•1 kg is shown.
The impulse at t=2 sec is –
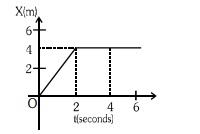
(1) 0•2 kgms–1
(2) –0•2 kgms–1
(3) 0•1 kgms–1
(4) –0•4 kgms–1
5. Fig shows, a battery is connected to screw with the help of conducting wire. A magnetic needle is placed near the screw.
(i) If screw is made of Cu then deviation in needle is x.
(ii) If screw is made of Al then deviation in needle is y.
(iii) A new screw is made whose resistance is equal to parallel combination of two screws made of Cu and Al. If this screw is used in circuit then deviation in magnetic needle is z.
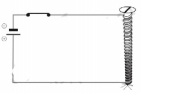
Choose correct option according to above given statements.
(1) x > y > z
(2) x > z > y
(3) z > x > y
(4) x = y = z
6. Ohm's law is
(1) a fundamental law.
(2) an empirical law.
(3) both fundamental and empirical law.
(4) neither fundamental nor empirical law.
7. A block of mass 10 kg is released on a fixed wedge Take initial velocity of block zero. Then work done by normal reaction (with respect to ground )on block in two second will be: (g = 10 m/s2).
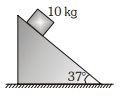
(1) zero
(2) 960 J
(3) 1200 J
(4) none of these
8. An alpha particle enters into a uniform magnetic field as shown in the figure below. The alpha
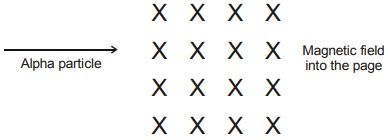
(1) not deflect when it enters into the magnetic field.
(2) deflects out of the page opposite to the direction of magnetic field.
(3) deflects towards top of the page as it enters into the magnetic field.
(4) deflects towards bottom of the page as it enters into the magnetic field.
9. Mass of a body on earth is 100 kg. Its mass at centre of earth is :
(1) 50 kg
(2) 25 kg
(3) Zero
(4) 100 kg
10. Unit of force is
(1) kg m/s
(2) Kg m-s2
(3) Kg m/s2
(4) Kg m-s
INTEGER
11. A cylindrical wire of resistance ‘R’ is pulled to thrice its length keeping the volume constant. It’s new resistance will be xR. What is the value of x ?
12. If a bar magnet is cut lengthwise into 3 parts, the total no. of poles will be 2x, then what will be the value of x.
13. A man standing on a cliff claps his hand hears its echo after 1 sec. If sound is reflected from another mountain and velocity of sound in air is 340 m/sec. Find the distance between the man and reflection point in meters .
14. Starting at rest, a 5 kg object is acted upon by only one force as indicated in figure. the total work done by the force is 10 × n J .Find the value of n.
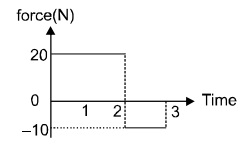
15. A cat runs 200 m away from a wall in a straight line in 12 sec. and then runs halfway back in two-third the time it took during forward motion. Calculate its average speed in m/s.
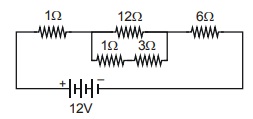
16. The current flowing through the 3: resistor in the electric circuit shown below is 3 × Z x 10–2 A. The value of Z is
17. A player caught a cricket ball of mass 200g which came to his hand with a speed of 20m/s. If the ball was stopped in 0.1s, the force exerted by ball on the hands of player is:
18. A bar magnet is cut into 10 equal pieces. The number of poles each piece is having is given by 2x. Find 'x'.
19. The gravitational force between the two objects is 4 N. Now if masses of both objects are halved without changing distance between them, then the new force of gravitation between the two is ............. N ?
20. One metre length of wire carries a constant current. The wire is bent to form a circular loop. The magnetic field at the centre of this loop is B. The same is now bent to form a circular loop of smaller radius to have four turns in the loop. The magnetic field at the centre of this new loop is nB. Find 'n'.
Click Here For Download Full Paper
Section -B : Chemistry Objective
21. Which of the following is having mass 64g?
(1) 2O2
(2) SO2
(3) 4O
(4) All of these
22. Arrange the following in the order of isobar, metals, isotopes , non-metals and noble gases.
(A) zAx, z+3Bx
(B) 2X4 ,18Y40
(C) zAx+4 ,zAx
(D) 8X16 ,15Y31
(E) 11A23 , 19Y39
(1) ABDEC
(2) CDEAB
(3) BDACE
(4) AECDB
23. The followings are the ions obtained by the dissociation of different salts, Identify the one that is not possible.
(1) Sodium potassium sulphate Na+, K+, SO2–4
(2) Bleaching powder Ca2+, Cl–, OCl–
(3) Potash alum K+, Al3+, SO2–4
(4) Sodium oxalate Na+, CO2–, CO–
24. What will be the product of this reaction
Zn + 2NaOH
(1) Zn(OH)2 + H2
(2) Na2ZnO2 + H2
(3) Zn(OH)2 + Na2
(4) Na2ZnO2 + Na2O
25. A water insoluble substance ‘X’ on reacting with dilute H2SO4 released a colourless & Odourless gas ‘Y’. On bubbling the gas through lime water it initially became milky due to the formation of ‘Z’ and the milkness disappeared when the gas pass in excess & form ‘R’. X, Y, Z & R are respectively –
(1) CaCO3, H2CO3, CO2, Ca(HCO3)2
(2) CaO, CaCO3, CO2(HCO3)2
(3) CaCO3, CO2, Ca(HCO3)2, Ca(OH)2
(4) CaCO3, CO2, CaCO3, Ca(HCO3)2
26. A gas produced in Chlor–alkali process is passed through dry slaked lime to prouduced a compound. This compound is represented as:
(1) CaH2
(2) Ca2+Cl–(OCl–)
(3) Ca2+O2–(Cl2)
(4) Ca2+(H–)(OH–)
27. Which of the following are exothermic processes
(i) Mixing of water with sulphuric acid
(ii) Decomposition of lime stone.
(iii) Condensation of water vapour
(iv) Sublimation of napthaline crystals
(1) i and ii
(2) ii and iii
(3) i and iv
(4) i and iii
28. Two elements X and Y have atomic weights of 14 and 16 respectively. They form a series of compounds A, B, C, D and E in which for the same amount of element X, Y is present in the ratio 1 : 2 : 3 : 4 : 5. If the compound A has 28 parts by weight of X and 16 parts by weight of Y, then the compound of E will have 28 parts by weight of X and-
(1) 32 parts by weight of Y
(2) 48 parts by weight of Y
(3) 64 parts by weight of Y
(4) 80 parts by weight of Y
29. The green coating on copper metal is generally cleaned with tamarind juice. It is becasue:
(1) Metal present in tamarind juice cause copper metal dispalcement
(2) It reacts with acidic CO2 of metal carbonate
(3) It reacts with basic metal oxide layer on the surface
(4) It reacts with basic metal carbonate layer on the surface.
30. Mg+2 and F– ions differ in which of the following fundamental particles?
(1) Electrons, protons and neutrons
(2) Protons and neutrons
(3) Only electrons
(4) Electrons and protons
INTEGER
31. How many acids are dibasic among the following
(i) HCl
(ii) H2SO4
(iii) H3PO4
(iv) H2CO3
(v) H3PO3
(vi) HCOOH
32. Identify the number of statements which are incorrect :
(i) 24 g of ozone contains 2 moles of ozone.
(ii) 1.7 f of ammonia contains 6.022 × 1020 molecules. (iii) 320 g of NaOH contains 48.17 × 1023 molecules.
(iv) 3 molecules of magnesium bicarbonate contain 3 atoms and magnesium, 6 atoms of hydrogen, 6 atoms of carbon and 6 atoms of oxygen.
(v) Ratio of mass of each atom in aluminium sulphite is 9 : 16 : 24.
(vi) 1 mole of water contains 12.044 × 1023 total number of electrons and protons.
33. How many of the following statements are correct?
(i) Plum Pudding model was given by scientist Dalton.
(ii) Name electron was given by scientist J. J. Thomson.
(iii) Neutrons were discovered by Chadwick.
(iv) Alpha particle is 2 times heavier than one proton.
(v) Rutherford gave concept of stationary orbit.
(vi) Isotopes have same physical properties and different chemical properties.
34. How many of the following can behave as only basic oxide:
(i) Na2O
(ii) MgO
(iii) Al2O3
(iv) K2O
(v) ZnO
(vi) BaO
(vii) Fe2O3
(viii) MnO
35. Acetic acid contains four hydrogen atoms, so its basicity is.
36. In the equation Na2CO3+ xHCl ------ 2NaCl + CO2+ H2O the value of x is
37. What would be the valency of an element whose atomic number is 16.
38. In the formula of ammonium sulphate total number of atoms are –
39. Identify no of incorrect chemical formula among the following: NaO, KOH, H2O2, CaP, AlN, LiClO4, Na2ZnO3, ZnHCO3, HNO2, CuI
40. An ion Y3– contains 10 electrons and 7 neutrons. What will be the atomic number of element Y
Click Here For Download Full Paper
Courtesy : CBSE
