(Download) CBSE Class-12 Sample Paper (Chemistry) 2014-15
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in

(Download) CBSE Class-12 Sample Paper (Chemistry) 2014-15
Time Allowed: 3 hr
Maximum marks: 70
1. The following figure shows the variation of adsorption of N2 on charcoal with pressure at different constant temperatures:
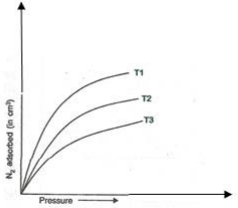
Arrange the temperatures T1, T2 and T3 in the increasing order.
2. Give the formula of a noble gas species which is isostructural with IBr2 -
.
3. What is the effect of synergic bonding interactions in a metal carbonyl
complex?
4. PCl5 acts as an oxidizing agent. Justify.
5. Write the name of the product formed when benzenediazonium chloride solution is treated with potassium iodide.
6. Name the crystal defect which reduces the density of an ionic solid? What type of ionic substances show this defect?
7. The molar conductivity ( λm ) of KCl solutions at different concentrations at 298 K is plotted as shown in the figure given below:
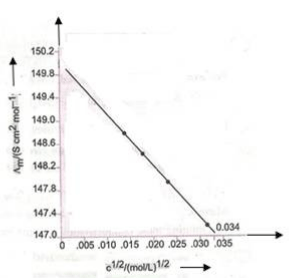
Determine the value of 0 λ m and A for KCl.
8. Aluminum crystallizes in anfcc structure. Atomic radius of the metal is 125 pm. What is the length of the side of the unit cell of the metal?
9. Draw the structure of the following compounds:
(i) H2S2O7
(ii) XeOF4
OR
Write balanced chemical equations for the following:
(i) Reaction of chlorine with hot and concentrated NaOH.
(ii) Sulphur dioxide is passed through an aqueous solution of Fe (III) salt.
Click Here To Download Full Sample Paper