CBSE Class-10 Exam 2016 : Marking Scheme, Science (Outside)
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in
CBSE Class-10 Exam 2016 : Marking Scheme, Science (Outside)
CBSE Class-10 Exam 2016 : Science (Outside) Set -1
SUMMATIVE ASSESSMENT - II
March 2016
Marking Scheme – Science (Outside Delhi) 31/1
March 2016
Marking Scheme – Science (Outside Delhi) 31/1
1. The Marking Scheme provides general guidelines to reduce subjectivity in the marking. It carries only suggested value points for the answer. These are only guidelines and do not constitute the complete answer. Any other individual response with suitable justification should also be accepted even if there is no reference to the text.
2. Evaluation is to be done as per instructions provided in the Marking Scheme. It should not be done according to one's own interpretation or any other consideration. Marking Scheme should be strictly adhered to and religiously followed.
3. If a question has parts, please award marks in the right hand side for each part. Marks awarded for different parts of the question should then be totalled up and written in the left hand margin.
2. Evaluation is to be done as per instructions provided in the Marking Scheme. It should not be done according to one's own interpretation or any other consideration. Marking Scheme should be strictly adhered to and religiously followed.
3. If a question has parts, please award marks in the right hand side for each part. Marks awarded for different parts of the question should then be totalled up and written in the left hand margin.
4. If a question does not have any parts, marks be awarded in the left hand side margin.
5. If a candidate has attempted an extra question, marks obtained in the question attempted first should be retained and the other answer should be scored out.
6. Wherever only two/three of a 'given' number of examples/factors/points are expected only the first two/three or expected number should be read. The rest are irrelevant and should not be examined.
7. There should be no effort at 'moderation' of the marks by the evaluating teachers. The actual total marks obtained by the candidate may be of no concern of the evaluators.
8. All the Head Examiners / Examiners are instructed that while evaluating the answer scripts, if the answer is found to be totally incorrect, the (X) should be marked on the incorrect answer and awarded ‘0’ marks.
9. ½ mark may be deducted if a candidate either does not write units or writes wrong units in the final answer of a numerical problem.
10. A full scale of mark 0 to 100 has to be used. Please do not hesitate to award full marks if the answer deserves it.
11. As per orders of the Hon’ble Supreme Court the candidates would now be permitted to obtain photocopy of the Answer Book on request on payment of the prescribed fee. All Examiners/Head Examiners are once again reminded that they must ensure that evaluation is carried out strictly as per value points given in the marking scheme.
5. If a candidate has attempted an extra question, marks obtained in the question attempted first should be retained and the other answer should be scored out.
6. Wherever only two/three of a 'given' number of examples/factors/points are expected only the first two/three or expected number should be read. The rest are irrelevant and should not be examined.
7. There should be no effort at 'moderation' of the marks by the evaluating teachers. The actual total marks obtained by the candidate may be of no concern of the evaluators.
8. All the Head Examiners / Examiners are instructed that while evaluating the answer scripts, if the answer is found to be totally incorrect, the (X) should be marked on the incorrect answer and awarded ‘0’ marks.
9. ½ mark may be deducted if a candidate either does not write units or writes wrong units in the final answer of a numerical problem.
10. A full scale of mark 0 to 100 has to be used. Please do not hesitate to award full marks if the answer deserves it.
11. As per orders of the Hon’ble Supreme Court the candidates would now be permitted to obtain photocopy of the Answer Book on request on payment of the prescribed fee. All Examiners/Head Examiners are once again reminded that they must ensure that evaluation is carried out strictly as per value points given in the marking scheme.
MARKING SCHEME
CLASS X – OUTSIDE DELHI
Code No. 31/1
Expected Answer/ Value point Marks Total
SECTION – A
CLASS X – OUTSIDE DELHI
Code No. 31/1
Expected Answer/ Value point Marks Total
SECTION – A
Q1. Propanol, ½, ½ 1
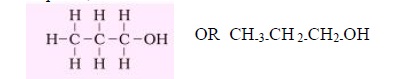
Q2. Its filament breaks up into smaller fragments or pieces, and each fragment grows into a new filament/individual. ½, ½ 1
Q3. Ultraviolet rays from the sun penetrate down the earth and cause health hazards/skin cancer in human beings 1 1
Q4.
- Concave Mirrors / Converging Mirrors ½
- When a solar furnace is placed at the focus of a large concave mirror/ reflector, it focuses a parallel beam of light on the furnace, consequently a high temperature is achieved after some time. 3 x ½ 2
Q5.
- Chipko Andolan (Hug the Trees Movement) – Women of Reni village in Garhwal hugged the tree trunks preventing the contractors from felling the trees. 1
- This Andolan quickly spread to other parts of the country and forced the government to rethink their priorities in the use of forest produce, consequently the local people benefitted.
- The environment was saved from permanent damage/ affected the quality of soil and the sources of water. ½, ½ 2
Q6. Burning of fossil fuels produces green house gases(CO , CO2,water vapour, oxides of nitrogen, sulphur). High concentration of CO2 causes global warming. 1, 1 2
Q7.
a) 2CH3COOH + 2Na → 2CH3COONa + H2 ½, ½
Sodium ethanoate/ Sodium acetate
b) CH3COOH + NaOH → CH3COONa + H2O ½, ½
Sodium ethanoate/ sodium acetate
c) CH3COOH +C2H5OH → CH3COOC2H5 + H2O ½, ½ 3
Ethyl ethanoate/ ester
a) 2CH3COOH + 2Na → 2CH3COONa + H2 ½, ½
Sodium ethanoate/ Sodium acetate
b) CH3COOH + NaOH → CH3COONa + H2O ½, ½
Sodium ethanoate/ sodium acetate
c) CH3COOH +C2H5OH → CH3COOC2H5 + H2O ½, ½ 3
Ethyl ethanoate/ ester
Click Here to Download Science (Outside) Set-1
Click Here to Download Science (Outside) Set-2
Click Here to Download Science (Outside) Set-3
Courtesy: CBSE
