(Download) CBSE Class-12 Sample Paper 2020-21:Chemistry
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in

(Download) CBSE Class-12 Sample Paper 2020-21 :Chemistry
SAMPLE PAPER 1
CHEMISTRY THEORY (043)
MM:70 Time: 3 Hours
General Instructions. Read the following instructions carefully.
a) There are 33 questions in this question paper. All questions are compulsory.
b) Section A: Q. No. 1 to 2 are case-based questions having four MCQs or Reason Assertion type based on given passage each carrying 1 mark.
c) Section A: Question 3 to 16 are MCQs and Reason Assertion type questions carrying 1 mark each
d) Section B: Q. No. 17 to 25 are short answer questions and carry 2 marks each. e) Section C: Q. No. 26 to 30 are short answer questions and carry 3 marks each. f) Section D: Q. No. 31 to 33 are long answer questions carrying 5 marks each. g) There is no overall choice. However, internal choices have been provided.
h) Use of calculators and log tables is not permitted.
SECTION A (OBJECTIVE TYPE)
1. Read the passage given below and answer the following questions: (1x4=4)
An efficient, aerobic catalytic system for the transformation of alcohols into carbonyl compounds under mild conditions, copper-based catalyst has been discovered. This copper-based catalytic system utilizes oxygen or air as the ultimate, stoichiometric oxidant, producing water as the only by-product
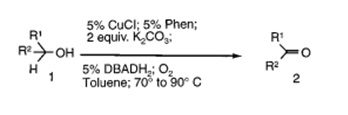
A wide range of primary, secondary, allylic, and benzylic alcohols can be smoothly oxidized to the corresponding aldehydes or ketones in good to excellent yields. Air can be conveniently used instead of oxygen without affecting the efficiency of the process. However, the use of air requires slightly longer reaction times.
This process is not only economically viable and applicable to large-scale reactions, but it is also environmentally friendly.
(Reference:Ohkuma, T., Ooka, H., Ikariya, T., & Noyori, R. (1995). Preferential hydrogenation of aldehydes and ketones. Journal of the American Chemical Society, 117(41), 10417-10418.)
The following questions are multiple choice questions. Choose the most appropriate answer:
(i)The Copper based catalyst mention in the study above can be used to convert:
a) propanol to propanonic acid b) propanone to propanoic acid c) propanone to propan-2-ol
d) propan-2-ol to propanone
(ii)The carbonyl compound formed when ethanol gets oxidised using this copper-based catalyst can also be obtained by ozonolysis of:
a) But-1-ene b) But-2-ene c) Ethene
d) Pent-1-ene
OR Which of the following is a secondary allylic alcohol? a) But-3-en-2-ol
b) But-2-en-2-ol c) Prop-2-enol
d) Butan-2-ol
(iii) Benzyl alcohol on treatment with this copper-based catalyst gives a compound ‘A’ which on reaction with KOH gives compounds ‘B’ and ‘C’. Compound ‘B’ on oxidation with KMnO4- KOH gives compound ‘C’. Compounds ‘A’, ‘B’ and ‘C’ respectively are :
a) Banzaldehyde, Benzyl alcohol, potassium salt of Benzoic acid b) Banzaldehyde, potassium salt of Benzoic acid, Benzyl alcohol c) Banzaldehyde, Benzoic acid, Benzyl alcohol
d) Benzoic acid, Benzyl alcohol, Benzaldehyde
(iv) An organic compound ‘X’ with molecular formula C3H8O on reaction with this copper based catalyst gives compound ‘Y’ which reduces Tollen’s reagent. ‘X’ on reaction with sodium metal gives ‘Z’ . What is the product of reaction of ‘Z’ with 2- chloro-2-methylpropane?
a) CH3CH2CH2OC(CH3)3
b) CH3CH2OC(CH3)3
c) CH2=C(CH3)2
d) CH3CH2CH=C(CH3)2
2. Read the passage given below and answer the following questions: (1x4=4)
The amount of moisture that leather adsorbs or loses is determined by temperature, relative humidity, degree of porosity, and the size of the pores. Moisture has great practical significance because its amount affects the durability of leather, and in articles such as shoes, gloves, and other garments, the comfort of the wearer. High moisture content accelerates deterioration and promotes mildew action. On the other hand, a minimum amount of moisture is required to keep leather properly lubricated and thus prevent cracking.
The study indicates that adsorption of moisture by leather is a multi-molecular process and is accompanied by low enthalpies of adsorption. Further 75-percent relative humidity the adsorption is a function of surface area alone. Untanned hide and chrome-tanned leathers have the largest surface areas. The leathers tanned with the vegetable tanning materials have smaller surface areas since they are composed of less hide substance and the capillaries are reduced to smaller diameters, in some cases probably completely filled by tanning materials. This process of tanning occurs due to mutual coagulation of positively charged hide with negatively charged tanning
