NCERT Chemistry Question Paper (Class - 12)
Disclaimer: This website is NOT associated with CBSE, for official website of CBSE visit - www.cbse.gov.in
NCERT Chemistry Question Paper (Class - 12)
:: Chapter 1 - The Solid State ::
INTEXT QUESTIONS
Question 1.1: Why are solids rigid?
Question 1.2: Why do solids have a definite volume?
Question 1.3: Classify the following as amorphous or crystalline solids: Polyurethane, naphthalene, benzoic acid, teflon, potassium nitrate, cellophane, olyvinylchloride, fibre glass, copper.
Question 1.4: Why is glass considered a super cooled liquid?
Question 1.5: Refractive index of a solid is observed to have the same value along all directions. Comment on the nature of this solid. Would it show cleavage property?
Question 1.6: Classify the following solids in different categories based on
the nature of intermolecular forces operating in them:
Potassium sulphate, tin, benzene, urea, ammonia, water, zinc sulphide, graphite,
rubidium, argon, silicon carbide.
Question 1.7: Solid A is a very hard electrical insulator in solid as well as in molten state and melts at extremely high temperature. What type of solid is it?
Question 1.8: Ionic solids conduct electricity in molten state but not in solid state. Explain.
Question 1.9: What type of solids are electrical conductors, malleable and ductile?
Question 1.10: Give the significance of a ‘lattice point’.
Question 1.11: Name the parameters that characterize a unit cell.
Question 1.12: Distinguish between (i) Hexagonal and monoclinic unit cells (ii) Face−centred and end−centred unit cells.
Question 1.13: Explain how much portion of an atom located at (i) corner and (ii) body−centre of a cubic unit cell is part of its neighboring unit cell.
Question 1.14: What is the two dimensional coordination number of a molecule in square close packed layer?
Question 1.15: A compound forms hexagonal close−packed structure. What is the total number of voids in 0.5 mol of it? How many of these are tetrahedral voids?
Question 1.16: A compound is formed by two elements M and N. The element N forms ccp and atoms of M occupy 1/3rd of tetrahedral voids. What is the formula of the compound?
Question 1.17: Which of the following lattices has the highest packing efficiency (i) simple cubic (ii) body−centred cubic and (iii) hexagonal close−packed lattice?
Question 1.18: An element with molar mass 2.7 × 10−2 kg mol−1 forms a cubic unit cell with edge length 405 pm. If its density is 2.7 × 103 kg m−3, what is the nature of the cubic unit cell?
Question 1.19: What type of defect can arise when a solid is heated? Which physical property is effected by it and in what way?
Question 1.20: What type of stoichiometric defect is shown by: (i) ZnS (ii) AgBr
Question 1.21: Explain how vacancies are introduced in an ionic solid when a cation of higher valence is added as an impurity in it.
Question 1.22: Ionic solids, which have anionic vacancies due to metal excess defect, develop colour.Explain with the help of a suitable example.
Question 1.23: A group 14 element is to be converted into n−type semiconductor by doping it with a suitable impurity. To which group should this impurity belong?
Question 1.24:What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your Solution:.
EXERCISE
Question 1:Define the term 'amorphous'. Give a few examples of amorphous solids.
Question 2. What makes a glass different from a solid such as quartz?
Question 3. Classify each of the following solids as ionic, metallic, molecular, network (covalent) or amorphous.
Question 4. (i) What is meant by the term 'coordination number' ? (ii) What is the coordination number of atoms (a) in a cubic close packed structure? (b) in a body–centered cubic structure?
Question 5. How can you determine the atomic mass of an unknown metal if you know its density?
Question 6. 'Stability of a crystal is reflected in the magnitude of its
melting points'. Comment.
Collect melting points of solid water, ethyl alcohol, diethyl ether and methane
from a data book. What can you say about the intermolecular forces between these
molecules?
Question 7. How will you distinguish between the following pairs of terms (i) Hexagonal close packing and cubic close packing (ii) Crystal lattice and unit cell (iii) Tetrahedral void and octahedral void.
Question 8 How many lattice points are there in one unit cell of each of the following lattice? (i) Face–centred cubic (ii) Face–centred tetragonal (iii) Body–centred
Question 9. Explain (i) The basis of similarities and differences between metallic and ionic crystals. (ii) Ionic solids are hard and brittle.
Question 10 Calculate the efficiency of packing in case of a metal crystal for (i) simple cubic (ii) body–centred cubic (iii) face–centred cubic (with the assumptions that atoms are touching each other).
Question 11 Silver crystallises in fcc lattice. If edge length of the cell is 4.07 × 10-8 cm and density is 0.5 g cm3, calculate the atomic mass of silver.
Question 12 A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body–centre. What is the formula of the compound? What are the coordination numbers of P and Q?
Question 13 Niobium crystallises in body–centred cubic structure. If density is 8.55 g cm–3, calculate atomic radius of niobium using its atomic mass 93 u.
Question 14 If the radius of the octahedral void is r and radius of the atoms in closepacking is R, derive relation between r and R.
Question 15 Copper crystallises into a fcc lattice with edge length 3.61 × 10–8 cm. Show that the calculated density is in agreement with its measured value of 8.92 g cm-3.
Question 16 Analysis shows that nickel oxide has the formula Ni0.98O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?
Question 17 What is a semiconductor? Describe the two main types of semiconductors and contrast their conduction mechanism.
Question 18: Non–stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1. Can you account for the fact that this substance is a p–type semiconductor?
Question 19: Ferric oxide crystallises in a hexagonal close–packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Question 20: Classify each of the following as being either a p–type or an n–type semiconductor: (i) Ge doped with In (ii) B doped with Si.
Question 21: Gold (atomic radius = 0.144 nm) crystallises in a face–centred unit cell. What is the length of a side of the cell?
Question 22: In terms of band theory, what is the difference (i) Between a conductor and an insulator (ii) Between a conductor and a semiconductor
Question 23: Explain the following terms with suitable examples: (i) Schottky defect (ii) Frenkel defect (iii) Interstitials and (iv) F–centres
Question 24: Aluminium crystallises in a cubic close–packed structure. Its metallic radius is 125 pm. (i) What is the length of the side of the unit cell? (ii) How many unit cells are there in 1.00 cm3 of aluminum?
Question 25: If NaCl is doped with 10−3 mol % of SrCl2, what is the concentration of cation vacancies?
Question 26: Explain the following with suitable examples: (i) Ferromagnetism (ii)Paramagnetism (iii)Ferrimagnetism (iv)Antiferromagnetism (v)12–16 and 13–15 group compounds.
:: Chapter 2 - Solutions ::
EXERCISE
2.2 Give an example of a solid solution in which the solute is a gas.
2.3 Define the following terms:
(i) Mole fraction
2.3 Define the following terms:
(ii) Molality
2.3 Define the following terms:
(iii) Molarity
2.3 Define the following terms:
(iv) Mass percentage.
2.4 Concentrated nitric acid used in laboratory work is 68% nitric acid by mass in aqueous solution. What should be the molarity of such a sample of the acid if the density of the solution is 1.504 g mL–1?
2.5 A solution of glucose in water is labelled as 10% w/w, what would be the molality and mole fraction of each component in the solution? If the density of solution is 1.2 g mL–1, then what shall be the molarity of the solution?
2.6 How many mL of 0.1 M HCl are required to react completely with 1 g mixture of Na2CO3 and NaHCO3 containing equimolar amounts of both?
2.7 A solution is obtained by mixing 300 g of 25% solution and 400 g of 40% solution by mass. Calculate the mass percentage of the resulting solution.
2.8 An antifreeze solution is prepared from 222.6 g of ethylene glycol (C2H6O2) and 200 g of water. Calculate the molality of the solution. If the density of the solution is 1.072 g mL–1, then what shall be the molarity of the solution?
:: Chapter 3 - Electrochemistry ::
CONCEPT
Electrochemistry and It's Uses
construction and functioning of Daniell cell
electrode potential, cell potential & Represent a galvanic cell
Structure and Working of Standard Hydrogen Electrode
measure the standard potential of Cu2+
Use of platinum or gold in standard hydrogen electrode
What is the Nernst Equation
Relation between EӨcell and Kc
Nernst Equation for the given chemical reaction
Gibbs energy of reaction taking place in an electrochemical cell
Conductance of Electrolytic Solutions
What is cell constant
What is a superconductor
Factor effecting conductance & ionic conductance
What are Electronically conducting polymers and there advantages
Problems takes place in measuring of conductivity
What is a conductivity cell
Method to measure conductance using Wheatstone bridge
Concept of Molar Conductivity
Kohlrausch law of independent migration of ions
How to measure equilibrium constant and limiting molar conductivity of week electrolytite
Faraday’s Laws of Electrolysis
Explain type of cells
What is corrosion explain how corrosion works as a cell
What is hydrogen economy
EXERCISE
Question 1:Arrange the following metals in the order in which they
displace each other from the solution of their salts.
Al, Cu, Fe, Mg and Zn
Question 2:Given the standard electrode potentials,
K+/K = −2.93V, Ag+/Ag = 0.80V,
Hg2+/Hg = 0.79V
Mg2+/Mg = −2.37 V, Cr3+/Cr = − 0.74V Arrange these metals
in their increasing order of reducing power.
Question 3:Depict the galvanic cell in which the reaction Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Question 4:Calculate the standard cell potentials of galvanic cells in which the following reactions take place:
(i) 2Cr(s) + 3Cd2+(aq) → 2Cr3+(aq) + 3Cd
(ii) Fe2+(aq) + Ag+(aq) → Fe3+(aq) + Ag(s)
Calculate the =∆rGθ and equilibrium constant of the reactions.
Question 5:Write the Nernst equation and emf of the following cells at 298 K:
(i) Mg(s) | Mg2+(0.001M) || Cu2+(0.0001 M) | Cu(s)
(ii) Fe(s) | Fe2+(0.001M) || H+(1M)|H2(g)(1bar)
| Pt(s)
(iii) Sn(s) | Sn2+(0.050 M) || H+(0.020 M) | H2(g)
(1 bar) | Pt(s)
(iv) Pt(s) | Br2(l) | Br−(0.010 M) || H
Question 6:In the button cells widely used in watches and other devices the following reaction takesplace:
Zn(s) + Ag2O(s) + H2O(l) → Zn2+(aq) + 2Ag(s) + 2OH−(aq) Determine and for the reaction.
Question 7:Define conductivity and molar conductivity for the solution of an electrolyte. Discuss their variation with concentration.
Question 8:The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 Scm−1. Calculate its molar conductivity.
Question 9:The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 500M. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10−3S cm−1.
Question 10:The conductivity of sodium chloride at 298 K has been determined at different concentrations and the results are given below:
Question 11: Conductivity of 0.00241 M acetic acid is 7.896 × 10−5 S cm−1. Calculate its molar conductivity and if for acetic acid is 390.5 S cm2 mol−1, what is its dissociation constant?
Question 12:How much charge is required for the following reductions:
(i) 1 mol of Al3+ to Al.
(ii) 1 mol of Cu2+ to Cu.
(iii) 1 mol of MnO4– to Mn2+.
Question 13:How much electricity in terms of Faraday is required to produce
(i) 20.0 g of Ca from molten CaCl2.
(ii) 40.0 g of Al from molten Al2O3.
Question 14: How much electricity is required in coulomb for the oxidation of (i) 1 mol of H2O to O2. (ii) 1 mol of FeO to Fe2O3.
Question 15: A solution of Ni(NO3)2 is electrolysed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Question 16:Three electrolytic cells A,B,C containing solutions of ZnSO4, AgNO3 and CuSO4, respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.4 g of silver deposited at the cathode of cell B. How long did the current flow? What mass of copper and zinc were deposited?
Question 17: Using the standard electrode potentials given in Table 3.1, predict if the reaction between the following is feasible:
(i) Fe3+(aq) and I−(aq)
(ii) Ag+ (aq) and Cu(s)
(iii) Fe3+ (aq) and Br−(aq)
(iv) Ag(s) and Fe3+(aq)
(v) Br2 (aq) and Fe2+ (aq).
Question 18: Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3with platinum electrodes.
(iii) A dilute solution of H2SO4with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
IN TEXT SOLUTION
Question 3.1: How would you determine the standard electrode potential of the
systemMg2+ | Mg?
Can you store copper sulphate solutions in a zinc pot?
Question 3.3:Consult the table of standard electrode potentials and suggest three substances that an oxidise ferrous ions under suitable conditions.
Question 3.4:Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Question 3.5: Calculate the emf of the cell in which the following reaction takes place:
Question 3.6: The cell in which the following reactions occurs:
Question 3.7: Why does the conductivity of a solution decrease with dilution?
Question 3.8:Suggest a way to determine the Λ°m value of water.
Question 3.9:The molar conductivity of 0.025 mol L−1 methanoic acid is 46.1 S cm2 mol−1. Calculate its degree of dissociation and dissociation constant. Given λ0(H+)= 349.6 S cm2 mol–1 and λ0(HCOO–) = 54.6 S cm2 mol–1
Question 3.10:If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons would flow through the wire?
Question 3.11:Suggest a list of metals that are extracted electrolytically.
Question 3.12:Consider the reaction:
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 8H2O
What is the quantity of electricity in coulombs needed to reduce 1 mol of
Cr2O72– ?
Question 3.14:Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Question 3.15: Explain how rusting of iron is envisaged as setting up of an electrochemical cell.
:: Chapter 4 - Chemical Kinetics ::
Question 4.11 The following results have been obtained during the kinetic studies of the reaction: 2A + B → C + D Determine the rate law and the rate constant for the reaction.
Question 4.12 The reaction between A and B is first order
with respect to A and zero order with respect to B. Fill in the blanks in the
following table:
Question 4.13 Calculate the half-life of a first order reaction from their rate
constants given below:
(i) 200 s–1
(ii) 2 min–1
(iii) 4 years–1
Question 4.14 The half-life for radioactive decay of 14C is 5730 years. An
archaeological artifact containing wood had only 80% of the 14C found in a
living tree. Estimate the age of the sample.
Question 4.15 The experimental data for decomposition of N2O5 [2N2O5 → 4NO2 +
O2] in gas phase at 318K are given below:
(i) Plot [N2O5] against t.
(ii) Find the half-life period for the reaction.
(iii) Draw a graph between log[N2O5] and t.
(iv) What is the rate law ?
(v) Calculate the rate constant.
(vi) Calculate the half-life period from k and compare it with (ii).
Question 4.16 The rate constant for a first order reaction is 60 s–1. How much
time will it take to reduce the initial concentration of the reactant to its
1/16th value?
Question 4.17 During nuclear explosion, one of the products is 90Sr with half-life of 28.1 years. If 1μg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, how much of it will remain after 10 years and 60 years if it is not lost metabolically.
Question 4.18 For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
Question 4.19 A first order reaction takes 40 min for 30% decomposition. Calculate t1/2.
Question 4.20 For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data are obtained. Calculate the rate constant.
Question 4.21 The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume. SO2Cl2 (g) → SO2 (g) + Cl2 (g)
Question 4.22 The rate constant for the decomposition of N2O5 at various temperatures is given below: Draw a graph between ln k and 1/T and calculate the values of A and Ea. Predict the rate constant at 30° and 50°C.
Question 4.23 The rate constant for the decomposition of hydrocarbons is 2.418 × 10–5s–1 at 546 K. If the energy of activation is 179.9 kJ/mol, what will be the value of pre-exponential factor.
Question 4.24 Consider a certain reaction A → Products with k = 2.0 × 10–2s–1. Calculate the concentration of A remaining after 100 s if the initial concentration of A is 1.0 mol L–1.
Question 4.25 Sucrose decomposes in acid solution into glucose and fructose according to the first order rate law, with t1/2 = 3.00 hours. What fraction of sample of sucrose remains after 8 hours ?
Question 4.26 The decomposition of hydrocarbon follows the equation k = ( 4.5 × 1011s–1) e-28000K/T Calculate Ea.
Question 4.27 The rate constant for the first order decomposition of H2O2 is given by the following equation: log k = 14.34 – 1.25 × 104K/T Calculate Ea for this reaction and at what temperature will its half-period be 256 minutes?
Question 4.28 The decomposition of A into product has value of k as4.5 × 103 s–1 at 10°C and energy of activation 60 kJ mol–1. At what temperature would k be 1.5 × 104s–1?
Question 4.29 The time required for 10% completion of a first order reaction at 298K is equal to that required for its 25% completion at 308K. If the value of A is 4 × 1010s–1. Calculate k at 318K and Ea.
Question 4.30 The rate of a reaction quadruples when the temperature changes from 293 K to 313 K. Calculate the energy of activation of the reaction assuming that it does not change with temperature.
:: Chapter 5 - Surface Chemistry ::
Question 5.1 Distinguish between the meaning of the terms
adsorption and absorption. Give one example of each.
Question 5.2 What is the difference between physisorption and chemisorption?
Question 5.3 Give reason why a finely divided substance is more effective as an adsorbent.
Question 5.4 What are the factors which influence the adsorption of a gas on a solid?
Question 5.5 What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Question 5.6 What do you understand by activation of adsorbent? How is it achieved?
Question 5.7 What role does adsorption play in heterogeneous catalysis?
Question 5.8 Why is adsorption always exothermic ?
Question 5.9 How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
Question 5.10 Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Question 5.11 What are lyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated ?
Question 5.12 What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids
Question 5.13 What are enzymes ? Write in brief the mechanism of enzyme catalysis.
Question 5.14 How are colloids classified on the basis of (i) physical states of components (ii) nature of dispersion medium and (iii) interaction between dispersed phase and dispersion medium
Question 5.15 Explain what is observed
(i) when a beam of light is passed through a colloidal sol.
(ii) an electrolyte, NaCl is added to hydrated ferric oxide sol.
(iii) electric current is passed through a colloidal sol?
Question 5.16 What are emulsions? What are their different types? Give example of each type.
Question 5.17 What is demulsification? Name two demulsifiers.
Question 5.18 Action of soap is due to emulsification and micelle formation. Comment.
Question 5.19 Give four examples of heterogeneous catalysis.
Question 5.20 What do you mean by activity and selectivity of catalysts?
Question 5.21 Describe some features of catalysis by zeolites.
Question 5.22 What is shape selective catalysis?
Question 5.23 Explain the following terms:
(i) Electrophoresis
(ii) Coagulation
(iii) Dialysis
(iv) Tyndall effect.
Question 5.24 Give four uses of emulsions.
Question 5.25 What are micelles? Give an example of a micellers system.
Question 5.26 Explain the terms with suitable examples:
(i) Alcosol
(ii) Aerosol
(iii) Hydrosol.
Question 5.27 Comment on the statement that “colloid is not a substance but a state of substance”
:: Chapter 6 - General Principles and Processes of Isolation of Elements ::
Question 6.1 Copper can be extracted by hydrometallurgy but not zinc. Explain.
Question 6.2 What is the role of depressant in froth floatation process?
Question 6.3 Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Question 6.4 Explain:
(i) Zone refining
(ii) Column chromatography.
Question 6.5 Out of C and CO, which is a better reducing agent at 673 K ?
Question 6.6 Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
Question 6.7 Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Question 6.8 Write chemical reactions taking place in the extraction of zinc from zinc blende.
Question 6.9 State the role of silica in the metallurgy of copper.
Question 6.10 What is meant by the term “chromatography”?
Question 6.11 What criterion is followed for the selection of the stationary phase in chromatography?
Question 6.12 Describe a method for refining nickel.
Question 6.13 How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
Question 6.14 Giving examples, differentiate between ‘roasting’ and ‘calcination’.
Question 6.15 How is ‘cast iron’ different from ‘pig iron”?
Question 6.16 Differentiate between “minerals” and “ores”.
Question 6.17 Why copper matte is put in silica lined converter?
Question 6.18 What is the role of cryolite in the metallurgy of aluminium ?
Question 6.19 How is leaching carried out in case of low grade copper ores?
Question 6.20 Why is zinc not extracted from zinc oxide through reduction using CO?
Question 6.21 The value of ΔfG0 for formation of Cr2 O3 is – 540 kJmol−1and that of Al2 O3 is – 827 kJmol−1. Is the reduction of Cr2 O3 possible with Al ?
Question 6.22 Out of C and CO, which is a better reducing agent for ZnO ?
Question 6.23 The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
Question 6.24 Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Question 6.25 What is the role of graphite rod in the electrometallurgy of aluminium?
Question 6.27 Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
Question 6.28 Predict conditions under which Al might be expected to reduce MgO. (Hint: See Intext question 6.4)
:: Chapter 7 - The p-Block Elements ::
Question 7.1 Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Question 7.2 Why does the reactivity of nitrogen differ from phosphorus?
Question 7.3 Discuss the trends in chemical reactivity of group 15 elements.
Question 7.4 Why does NH3 form hydrogen bond but PH3 does not?
Question 7.5 How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Question 7.6 How is ammonia manufactured industrially?
Question 7.7 Illustrate how copper metal can give different products on reaction with HNO3.
Question 7.8 Give the resonating structures of NO2 and N2O5.
Question 7.9 The HNH angle value is higher than HPH, HAsH and HSbH angles. Why? [Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s–p bonding between hydrogen and other elements of the group].
Question 7.10 Why does R3P = O exist but R3N = O does not (R = alkyl group)?
Question 7.11 Explain why NH3 is basic while BiH3 is only feebly basic.
Question 7.12 Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Question 7.13 Write main differences between the properties of white phosphorus and red phosphorus.
Question 7.14 Why does nitrogen show catenation properties less than phosphorus?
Question 7.15 Give the disproportionation reaction of H3PO3.
Question 7.16 Can PCl5 act as an oxidising as well as a reducing agent? Justify.
Question 7.17 Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Question 7.18 Why is dioxygen a gas but sulphur a solid?
Question 7.19 Knowing the electron gain enthalpy values for O → O– and O → O2– as –141 and 702 kJ mol–1 respectively, how can you account for the formation of a large number of oxides having O2– species and not O–? (Hint: Consider lattice energy factor in the formation of compounds).
Question 7.20 Which aerosols deplete ozone?
Question 7.21 Describe the manufacture of H2SO4 by contact process?
Question 7.22 How is SO2 an air pollutant?
Question 7.23 Why are halogens strong oxidising agents?
Question 7.24 Explain why fluorine forms only one oxoacid, HOF.
Question 7.25 Explain why inspite of nearly the same electronegativity, oxygen forms hydrogen bonding while chlorine does not.
Question 7.26 Write two uses of ClO2.
Question 7.27 Why are halogens coloured?
Question 7.28 Write the reactions of F2 and Cl2 with water.
Question 7.29 How can you prepare Cl2 from HCl and HCl from Cl2? Write reactions only.
Question 7.30 What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Question 7.31 What are the oxidation states of phosphorus in the following:
(i) H3PO3
(ii) PCl3
(iii) Ca3P2
(iv) Na3PO4
(v) POF3? Exercises Chemistry 208
Question 7.32 Write balanced equations for the following:
(i) NaCl is heated with sulphuric acid in the presence of
MnO2.
(ii) Chlorine gas is passed into a solution of NaI in water.
Question 7.33 How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Question 7.34 With what neutral molecule is ClO– isoelectronic? Is that molecule a Lewis base?
Question 7.35 How are XeO3 and XeOF4 prepared?
Question 7.36 Arrange the following in the order of property indicated for each set:
(i) F2, Cl2, Br2, I2 - increasing bond dissociation enthalpy.
(ii) HF, HCl, HBr, HI - increasing acid strength.
(iii) NH3, PH3, AsH3, SbH3, BiH3 – increasing base strength.
Question 7.37 Which one of the following does not exist?
(i) XeOF4
(ii) NeF2
(iii) XeF2
(iv) XeF6
Question 7.38 Give the formula and describe the structure of a noble gas species which is isostructural with:
(i) ICl4 –
(ii) IBr2 –
(iii) BrO3 –
Question 7.39 Why do noble gases have comparatively large atomic sizes?
Question 7.40 List the uses of neon and argon gases.
:: Chapter 8 - The d- and f- Block Elements ::
Question 8.1 Write down the electronic configuration of:
(i) Cr3+
(iii) Cu+
(v) Co2 +
(vii) Mn2+
(ii) Pm3+
(iv) Ce4+
(vi) Lu2+
(viii) Th4+
Question 8.2 Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state?
Question 8.3 Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
Question 8.4 To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
Question 8.5 What may be the stable oxidation state of the transition element with the following d electron configurations in the ground state of their atoms : 3d3, 3d5, 3d8 and 3d4?
Question 8.6 Name the oxometal anions of the first series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
Question 8.7 What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Question 8.8 What are the characteristics of the transition elements and why are they called transition elements? Which of the d-block elements may not be regarded as the transition elements?
Question 8.9 In what way is the electronic configuration of the transition elements different from that of the non transition elements?
Question 8.10 What are the different oxidation states exhibited by the lanthanoids?
Question 8.11 Explain giving reasons:
(i) Transition metals and many of their compounds show
paramagnetic behaviour.
(ii) The enthalpies of atomisation of the transition metals are high.
(iii) The transition metals generally form coloured compounds.
(iv) Transition metals and their many compounds act as good catalyst
Question 8.12 What are interstitial compounds? Why are such compounds well known for transition metals?
Question 8.13 How is the variability in oxidation states of transition metals different from that of the non transition metals? Illustrate with examples.
Question 8.14 Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Question 8.15 Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with: (i) iodide (ii) iron(II) solution and (iii) H2S Exercises 235 The d- and f- Block Elements
Question 8.16 Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with (i) iron(II) ions (ii) SO2 and (iii) oxalic acid? Write the ionic equations for the reactions.
Question 8.17 For M2+/M and M3+/M2+ systems the EV values for some metals are as follows: Cr2+/Cr -0.9V Cr3/Cr2+ -0.4 V Mn2+/Mn -1.2V Mn3+/Mn2+ +1.5 V Fe2+/Fe -0.4V Fe3+/Fe2+ +0.8 V Use this data to comment upon: (i) the stability of Fe3+ in acid solution as compared to that of Cr3+ or Mn3+ and (ii) the ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Question 8.18 Predict which of the following will be coloured in aqueous solution? Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ and Co2+. Give reasons for each.
Question 8.19 Compare the stability of +2 oxidation state for the elements of the first transition series.
Question 8.20 Compare the chemistry of actinoids with that of the lanthanoids with special reference to: (i) electronic configuration (iii) oxidation state (ii) atomic and ionic sizes and (iv) chemical reactivity.
Question 8.21 How would you account for the following:
(i) Of the d4 species, Cr2+ is strongly reducing while
manganese(III) is strongly oxidising.
(ii) Cobalt(II) is stable in aqueous solution but in the presence of complexing
reagents it is easily oxidised.
(iii) The d1 configuration is very unstable in ions.
Question 8.22 What is meant by ‘disproportionation’? Give two examples of disproportionation reaction in aqueous solution.
Question 8.23 Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why?
Question 8.24 Calculate the number of unpaired electrons in the following gaseous ions: Mn3+, Cr3+, V3+ and Ti3+. Which one of these is the most stable in aqueous solution?
Question 8.25 Give examples and suggest reasons for the following features of the transition metal chemistry
(i) The lowest oxide of transition metal is basic, the
highest is amphoteric/acidic.
(ii) A transition metal exhibits highest oxidation state in oxides and
fluorides.
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
Question 8.26 Indicate the steps in the preparation of:
(i) K2Cr2O7 from chromite ore.
(ii) KMnO4 from pyrolusite ore.
Question 8.27 What are alloys? Name an important alloy which contains some of the lanthanoid metals. Mention its uses.
Question 8.28 What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements : 29, 59, 74, 95, 102, 104.
Question 8.29 The chemistry of the actinoid elements is not so smooth as that of the lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
Question 8.30 Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
:: Chapter 9 - Coordination Compounds ::
Question 9.1 Explain the bonding in coordination compounds in terms of Werner’s postulates.
Question 9.2 FeSO4 solution mixed with (NH4)2SO4 solution in 1:1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of Cu2+ ion. Explain why?
Question 9.3 Explain with two examples each of the following: coordination entity, ligand, coordination number, coordination polyhedron, homoleptic and heteroleptic.
Question 9.4 What is meant by unidentate, didentate and ambidentate ligands? Give two examples for each.
Question 9.5 Specify the oxidation numbers of the metals
in the following coordination entities:
(i) [Co(H2O)(CN)(en)2]2+
(ii) [CoBr2(en)2]+
(iii) [PtCl4]2–
(iv) K3[Fe(CN)6]
(v) [Cr(NH3)3Cl3 ]
Question 9.6 Using IUPAC norms write the formulas for the
following:
(i) Tetrahydroxozincate(II)
(ii) Potassium tetrachloridopalladate(II)
(iii) Diamminedichloridoplatinum(II)
(iv) Potassium tetracyanonickelate(II)
(v) Pentaamminenitrito-O-cobalt(III)
(vi) Hexaamminecobalt(III) sulphate
(vii) Potassium tri(oxalato)chromate(III)
(viii) Hexaammineplatinum(IV)
(ix) Tetrabromidocuprate(II)
(x) Pentaamminenitrito-N-cobalt(III)
Question 9.7 Using IUPAC norms write the systematic names
of the following:
(i) [Co(NH3)6]Cl3
(ii) [Pt(NH3)2Cl(NH2CH3)]Cl
(iii) [Ti(H2O)6]3+
(iv) [Co(NH3)4Cl(NO2)]Cl
(v) [Mn(H2O)6]2+
(vi) [NiCl4]2–
(vii) [Ni(NH3)6]Cl2
(viii) [Co(en)3]3+
(ix) [Ni(CO)4]
Question 9.8 List various types of isomerism possible for coordination compounds, giving an example of each.
Question 9.9 How many geometrical isomers are possible in
the following coordination entities?
(i) [Cr(C2O4)3]3–
(ii) [Co(NH3)3Cl3]
Question 9.10 Draw the structures of optical isomers of:
(i) [Cr(C2O4)3]3– (ii) [PtCl2(en)2]2+
(iii) [Cr(NH3)2Cl2(en)]+ 259 Coordination Compounds
Question 9.11 Draw all the isomers (geometrical and
optical) of:
(i) [CoCl2(en)2]+
(ii) [Co(NH3)Cl(en)2]2+
(iii) [Co(NH3)2Cl2(en)]+
Question 9.12 Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how many of these will exhibit optical isomers?
Question 9.13 Aqueous copper sulphate solution (blue in colour) gives: (i) a green precipitate with aqueous potassium fluoride and (ii) a bright green solution with aqueous potassium chloride. Explain these experimental results.
Question 9.14 What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is passed through this solution?
Question 9.15 Discuss the nature of bonding in the
following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4–
(ii) [FeF6]3–
(iii) [Co(C2O4)3]3–
(iv) [CoF6]3–
Question 9.16 Draw figure to show the splitting of d orbitals in an octahedral crystal field.
Question 9.17 What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
Question 9.18 What is crystal field splitting energy? How does the magnitude of Δo decide the actual configuration of d orbitals in a coordination entity?
Question 9.19 [Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2– is diamagnetic. Explain why?
Question 9.20 A solution of [Ni(H2O)6]2+ is green but a solution of [Ni(CN)4]2– is colourless. Explain.
Question 9.21 [Fe(CN)6]4– and [Fe(H2O)6]2+ are of different colours in dilute solutions. Why?
Question 9.22 Discuss the nature of bonding in metal carbonyls.
Question 9.23 Give the oxidation state, d orbital
occupation and coordination number of the central metal ion in the following
complexes:
(i) K3[Co(C2O4)3]
(iii) (NH4)2[CoF4]
(ii) cis-[Cr(en)2Cl2]C l
(iv) [Mn(H2O)6]SO4
Question 9.24 Write down the IUPAC name for each of the
following complexes and indicate the oxidation state, electronic configuration
and coordination number. Also give stereochemistry and magnetic moment of the
complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O
(ii) [Co(NH3)5Cl-]Cl2
(iii) CrCl3(py)3
(iv) Cs[FeCl4]
(v) K4[Mn(CN)6]
Question 9.25 What is meant by stability of a coordination compound in solution? State the factors which govern stability of complexes.
Question 9.26 What is meant by the chelate effect? Give an example. 9
Question 9.27 Discuss briefly giving an example in each
case the role of coordination compounds in:
(i) biological systems
(iii) analytical chemistry
(ii) medicinal chemistry
(iv) extraction/metallurgy of metals.
Question 9.28 How many ions are produced from the complex
Co(NH3)6Cl2 in solution?
(i) 6
(ii) 4
(iii) 3
(iv) 2
Question 9.29 Amongst the following ions which one has
the highest magnetic moment value?
(i) [Cr(H2O)6]3+
(ii) [Fe(H2O)6]2+
(iii) [Zn(H2O)6]2+
Question 9.30 The oxidation number of cobalt in
K[Co(CO)4] is
(i) +1
(ii) +3
(iii) –1
(iv) –3
:: Chapter 10 - Haloalkanes and Haloarenes ::
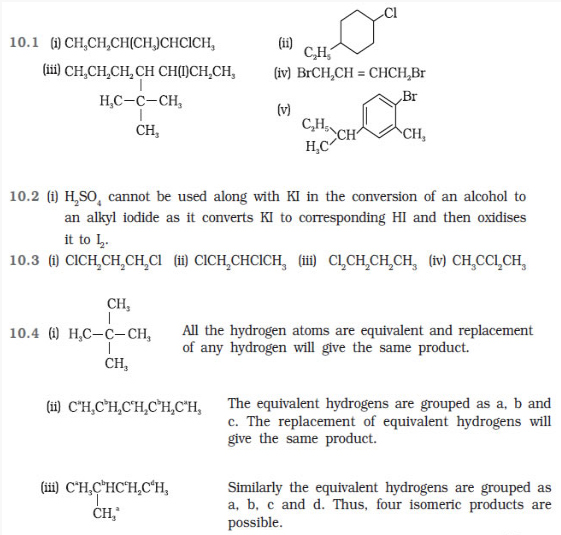
Question 10.1 Name the following halides according to
IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary,
tertiary), vinyl or aryl halides:
(i) (CH3)2CHCH(Cl)CH3
(ii) CH3CH2CH(CH3)CH(C2H5)Cl
(iii) CH3CH2C(CH3)2CH2I
(iv) (CH3)3CCH2CH(Br)C6H5
(v) CH3CH(CH3)CH(Br)CH3
(vi) CH3C(C2H5)2CH2Br
(vii) CH3C(Cl)(C2H5)CH2CH3
(viii) CH3CH=C(Cl)CH2CH(CH3)2
(ix) CH3CH=CHC(Br)(CH3)2
(x) p-ClC6H4CH2CH(CH3)2
(xi) m-ClCH2C6H4CH2C(CH3)3
(xii) o-Br-C6H4CH(CH3)CH2CH3
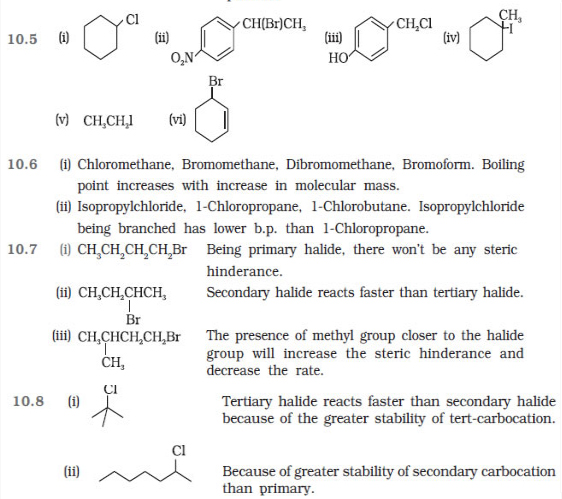
Question 10.2 Give the IUPAC names of the following
compounds:
(i) CH3CH(Cl)CH(Br)CH3
(ii) CHF2CBrClF
(iii) ClCH2C≡CCH2Br (iv) (CCl3)3CCl
(v) CH3C(p-ClC6H4)2CH(Br)CH3
(vi) (CH3)3CCH=ClC6H4I-p
Question 10.3 Write the structures of the following
organic halogen compounds.
(i) 2-Chloro-3-methylpentane
(ii) p-Bromochlorobenzene
(iii) 1-Chloro-4-ethylcyclohexane
(iv) 2-(2-Chlorophenyl)-1-iodooctane
(v) Perfluorobenzene
(vi) 4-tert-Butyl-3-iodoheptane
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
(viii) 1,4-Dibromobut-2-ene
Question 10.4 Which one of the following has the highest
dipole moment?
(i) CH2Cl2
(ii) CHCl3
(iii) CCl4
Question 10.5 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Question 10.6 Write the isomers of the compound having formula C4H9Br.
Question 10.7 Write the equations for the preparation of
1-iodobutane from
(i) 1-butanol
(ii) 1-chlorobutane
(iii) but-1-ene.
Question 10.8 What are ambident nucleophiles? Explain with an example.
Question 10.9 Which compound in each of the following
pairs will react faster in SN2 reaction with –OH?
(i) CH3Br or CH3I
(ii) (CH3)3CCl or CH3Cl
Question 10.10 Predict all the alkenes that would be
formed by dehydrohalogenation of the following halides with sodium ethoxide in
ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane
(ii) 2-Chloro-2-methylbutane
(iii) 2,2,3-Trimethyl-3-bromopentane.
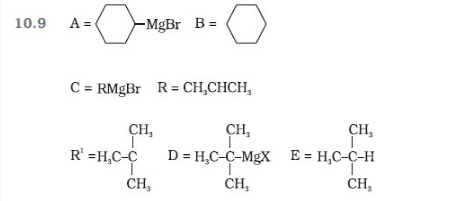
Question 10.11 How will you bring about the following
conversions?
(i) Ethanol to but-1-yne
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromomethane to propanone
(viii) But-1-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl.
Question 10.12 Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl
chloride?
(ii) alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions? 1
Question 10.13 Give the uses of freon 12, DDT, carbon tetrachloride and iodoform. 1
Question 10.14 Write the structure of the major organic product in each of the following reactions:
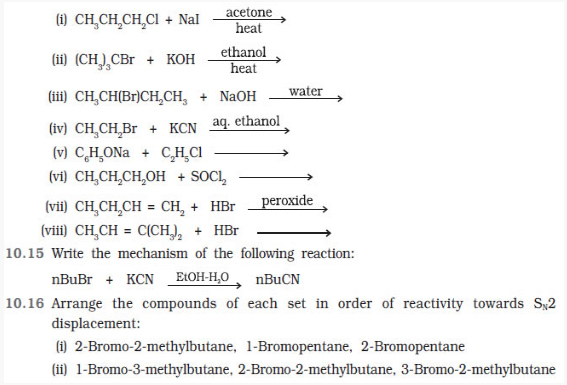
(iii) 1-Bromobutane, 1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane.
Question 10.17 Out of C6H5CH2Cl and C6H5CHClC6H5, which is more easily hydrolysed by aqueous KOH?
Question 10.18 p-Dichlorobenzene has higher m.p. and solubility than those of o- and m-isomers. Discuss.
Question 10.19 How the following conversions can be
carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chloropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
Question 10.20 The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Question 10.21 Primary alkyl halide C4H9Br
(a) reacted with alcoholic KOH to give compound (b). Compound (b) is reacted
with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium
metal it gives compound (d), C8H18 which is different from the compound formed
when n-butyl bromide is reacted with sodium. Give the structural formula of (a)
and write the equations for all the reactions.
Question 10.22 What happens when
(i) n-butyl chloride is treated with alcoholic KOH
(ii) bromobenzene is treated with Mg in the presence of dry ether,
(iii) chlorobenzene is subjected to hydrolysis,
(iv) ethyl chloride is treated with aqueous KOH,
(v) methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN?
:: Chapter 11 - Alcohols, Phenols and Ethers ::
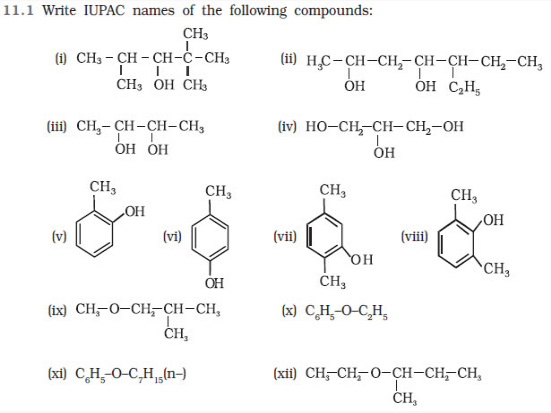
Question 11.2 Write structures of the compounds whose IUPAC names are as
follows:
(i) 2-Methylbutan-2-ol
(ii) 1-Phenylpropan-2-ol
(iii) 3,5-Dimethylhexane –1, 3, 5-triol (iv) 2,3 – Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan-3-ol
(ix) Cyclopent-3-en-1-ol
(x) 3-Chloromethylpentan-1-ol. 1
Question 11..3 (i) Draw the structures of all isomeric alcohols of molecular formula C5H12O and give their IUPAC names. (ii) Classify the isomers of alcohols in question
Question 11.3 (i) as primary, secondary and tertiary alcohols.
Question 11.4 Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Question 11.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Question 11.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Question 11.7 Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Question 11.8 While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Question 11.9 Give the equations of reactions for the preparation of phenol from cumene.
Question 11.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
Question 11.11 Write the mechanism of hydration of ethene to yield ethanol.
Question 11.12 You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
Question 11.13 Show how will you synthesise:
(i) 1-phenylethanol from a suitable alkene .
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) pentan-1-ol using a suitable alkyl halide?
Question 11.14 Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Question 11.15 Explain why is ortho nitrophenol more acidic than ortho methoxyphenol ?
Question 11.16 Explain how does the –OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Question 11.17 Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline KMnO4 solution.
(ii) Bromine in CS2 with phenol.
(iii) Dilute HNO3 with phenol.
(iv) Treating phenol wih chloroform in presence of aqueous NaOH.
Question 11.18 Explain the following with an example.
(i) Kolbe’s reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
Question 11.19 Write the mechanism of acid dehydration of ethanol to yield
ethene.
Question 11.20 How are the following conversions carried out?
(i) Propene → Propan-2-ol.
(ii) Benzyl chloride → Benzyl alcohol.
(iii) Ethyl magnesium chloride → Propan-1-ol.
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol.
Question 11.21 Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Bromination of phenol to 2,4,6-tribromophenol.
(iv) Benzyl alcohol to benzoic acid. (v) Dehydration of propan-2-ol to propene.
(vi) Butan-2-one to butan-2-ol.
Question 11.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
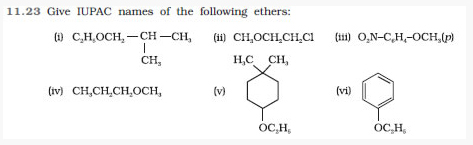
Question 11.24 Write the names of reagents and equations for the
preparation of the following ethers by Williamson’s synthesis:
(i) 1-Propoxypropane
(ii) Ethoxybenzene
(iii) 2-Methoxy-2-methylpropane
(iv) 1-Methoxyethane
Question 11.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Question 11.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Question 11.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Question 11.28 Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane (ii) methoxybenzene and (iii) benzyl ethyl ether.
Question 11.29 Explain the fact that in aryl alkyl ethers
(i) the alkoxy group activates the benzene ring towards electrophilic
substitution and (ii) it directs the incoming substituents to ortho and para
positions in benzene ring.
Question 11.30 Write the mechanism of the reaction of HI with methoxymethane.
Question 11.31 Write equations of the following reactions:
(i) Friedel-Crafts reaction – alkylation of anisole.
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium.
(iv) Friedel-Craft’s acetylation of anisole.
Question 11.32 Show how would you synthesise the following alcohols from appropriate
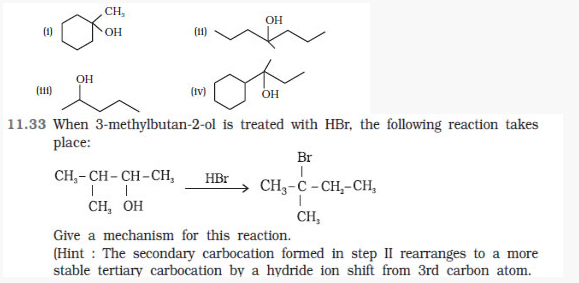
:: Chapter 12 - Aldehydes, Ketones and Carboxylic Acids ::
Question 12.1 What is meant by the following terms ? Give an example of the reaction in each case.
(i) Cyanohydrin
(ii) Acetal
(iii) Semicarbazone
(iv) Aldol
(v) Hemiacetal
(vi) Oxime
(vii) Ketal
(vii) Imine
(ix) 2,4-DNP-derivative
(x) Schiff’s base
Question 12.2 Name the following compounds according to IUPAC system of nomenclature:
(i) CH3CH(CH3)CH2CH2CHO
(ii) CH3CH2COCH(C2H5)CH2CH2Cl
(iii) CH3CH=CHCHO
(iv) CH3COCH2COCH3
(v) CH3CH(CH3)CH2C(CH3)2COCH3
(vi) (CH3)3CCH2COOH
(vii) OHCC6H4CHO-p
Question 12.3 Draw the structures of the following compounds.
(i) 3-Methylbutanal
(ii) p-Nitropropiophenone
(iii) p-Methylbenzaldehyde
(iv) 4-Methylpent-3-en-2-one
(v) 4-Chloropentan-2-one
(vi) 3-Bromo-4-phenylpentanoic acid
(vii) p,p’-Dihydroxybenzophenone
(viii) Hex-2-en-4-ynoic acid
Question 12.4 Write the IUPAC names of the following ketones and aldehydes. Wherever possible, give also common names.
(i) CH3CO(CH2)4CH3
(ii) CH3CH2CHBrCH2CH(CH3)CHO
(iii) CH3(CH2)5CHO
(iv) Ph-CH=CH-CHO
Question 12.5 Draw structures of the following derivatives.
(i) The 2,4-dinitrophenylhydrazone of benzaldehyde
(ii) Cyclopropanone oxime
(iii) Acetaldehydedimethylacetal
(iv) The semicarbazone of cyclobutanone
(v) The ethylene ketal of hexan-3-one
(vi) The methyl hemiacetal of formaldehyde
Question 12.6 Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents.
(i) PhMgBr and then H3O+
(ii) Tollens’ reagent
(iii) Semicarbazide and weak acid
(iv) Excess ethanol and acid
(v) Zinc amalgam and dilute hydrochloric acid
Question 12.7 Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
(i) Methanal
(ii) 2-Methylpentanal
(iii) Benzaldehyde
(iv) Benzophenone
(v) Cyclohexanone
(vi) 1-Phenylpropanone
(vii) Phenylacetaldehyde
(viii) Butan-1-ol
(ix) 2,2-Dimethylbutanal
Question 12.8 How will you convert ethanal into the following compounds?
(i) Butane-1,3-diol
(ii) But-2-enal
(iii) But-2-enoic acid
Question 12.9 Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Question 12.10 An organic compound with the molecular formula C9H10O forms 2,4-DNP derivative, reduces Tollens’ reagent and undergoes Cannizzaro reaction. On vigorous oxidation, it gives 1,2-benzenedicarboxylic acid. Identify the compound.
Question 12.11 An organic compound
(A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
Question 12.12 Arrange the following compounds in increasing order of their property as indicated:
(i) Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl
ketone (reactivity towards HCN)
(ii) CH3CH2CH(Br)COOH, CH3CH(Br)CH2COOH, (CH3)2CHCOOH, CH3CH2CH2COOH (acid
strength)
(iii) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid,
4-Methoxybenzoic acid (acid strength)
Question 12.13 Give simple chemical tests to distinguish between the following pairs of compounds.
(i) Propanal and Propanone
(ii) Acetophenone and Benzophenone
(iii) Phenol and Benzoic acid
(iv) Benzoic acid and Ethyl benzoate
(v) Pentan-2-one and Pentan-3-one
(vi) Benzaldehyde and Acetophenone
(vii) Ethanal and Propanal
Question 12.14 How will you prepare the following compounds from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom
(i) Methyl benzoate
(ii) m-Nitrobenzoic acid
(iii) p-Nitrobenzoic acid
(iv) Phenylacetic acid
(v) p-Nitrobenzaldehyde.
Question 12.15 How will you bring about the following conversions in not more than two steps?
(i) Propanone to Propene
(ii) Benzoic acid to Benzaldehyde
(iii) Ethanol to 3-Hydroxybutanal
(iv) Benzene to m-Nitroacetophenone
(v) Benzaldehyde to Benzophenone
(vi) Bromobenzene to 1-Phenylethanol
(vii) Benzaldehyde to 3-Phenylpropan-1-ol
(viii) Benazaldehyde to α-Hydroxyphenylacetic acid
(ix) Benzoic acid to m- Nitrobenzyl alcohol
Question 12.16 Describe the following:
(i) Acetylation
(ii) Cannizzaro reaction
(iii) Cross aldol condensation
(iv) Decarboxylation
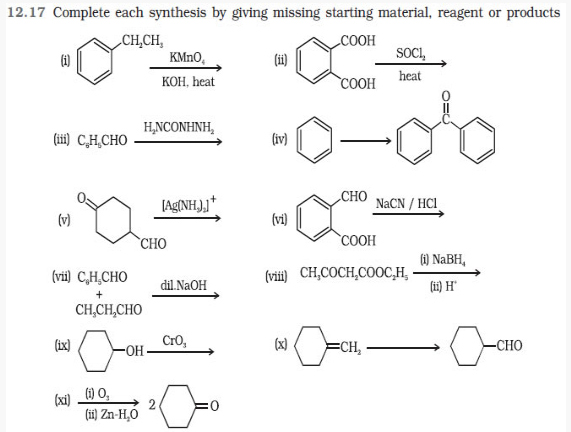
Question 12.18 Give plausible explanation for each of the following:
(i) Cyclohexanone forms cyanohydrin in good yield but
2,2,6-trimethylcyclohexanone does not.
(ii) There are two –NH2 groups in semicarbazide. However, only one is involved
in the formation of semicarbazones.
(iii) During the preparation of esters from a carboxylic acid and an alcohol in
the presence of an acid catalyst, the water or the ester should be removed as
soon as it is formed.
Question 12.19 An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acid. Write the possible structure of the compound.
Question 12.20 Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
:: Chapter 13 - Amines ::
Question 13.1 Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
(i) (CH3)2CHNH2
(ii) CH3(CH2)2NH2
(iii) CH3NHCH(CH3)2
(iv) (CH3)3CNH2
(v) C6H5NHCH3
(vi) (CH3CH2)2NCH3
(vii) m–BrC6H4NH2
Question 13.2 Give one chemical test to distinguish between the following
pairs of compounds.
(i) Methylamine and dimethylamine
(ii) Secondary and tertiary amines
(iii) Ethylamine and aniline
(iv) Aniline and benzylamine
(v) Aniline and N-methylaniline.
Question 13.3 Account for the following:
(i) pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not .
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated
ferric oxide.
(iv) Although amino group is o– and p– directing in aromatic electrophilic
substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable th an those of aliphatic
amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary
amines.
Question 13.4 Arrange the following:
(i) In decreasing order of the pKb values: C2H5NH2,
C6H5NHCH3, (C2H5)2NH and C6H5NH2
(ii) In increasing order of basic strength: C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and
CH3NH2
(iii) In increasing order of basic strength: (a) Aniline, p-nitroaniline and p-toluidine (b)
C6H5NH2, C6H5NHCH3, C6H5CH2NH2.
(iv) In decreasing order of basic strength in gas phase: C2H5NH2, (C2H5)2NH,
(C2H5)3N and NH3
(v) In increasing order of boiling point: C2H5OH, (CH3)2NH, C2H5NH2
(vi) In increasing order of solubility in water: C6H5NH2, (C2H5)2NH, C2H5NH2.
Question 13.5 How will you convert:
(i) Ethanoic acid into methanamine
(ii) Hexanenitrile into 1-aminopentane
(iii) Methanol to ethanoic acid
(iv) Ethanamine into methanamine
(v) Ethanoic acid into propanoic acid
(vi) Methanamine into ethanamine
(vii) Nitromethane into dimethylamine
(viii) Propanoic acid into ethanoic acid?
Question 13.6 Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Question 13.7 Write short notes on the following:
(i) Carbylamine reaction
(ii) Diazotisation
(iii) Hofmann’s bromamide reaction
(iv) Coupling reaction
(v) Ammonolysis
(vi) Acetylation
(vii) Gabriel phthalimide synthesis.
Question 13.8 Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
(ii) Benzene to m-bromophenol
(iii) Benzoic acid to aniline
(iv) Aniline to 2,4,6-tribromofluorobenzene
(v) Benzyl chloride to 2-phenylethanamine
(vi) Chlorobenzene to p-chloroaniline
(vii) Aniline to p-bromoaniline
(viii) Benzamide to toluene
(ix) Aniline to benzyl alcoho
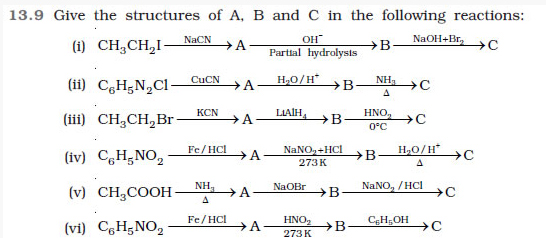
Question 13.10 An aromatic compound ‘A’ on treatment with aqueous ammonia and heating forms compound ‘B’ which on heating with Br2 and KOH forms a compound ‘C’ of molecular formula C6H7N. Write the structures and IUPAC names of compounds A, B and C.
Question 13.11 Complete the following reactions:
(i) C6H5NH2 + CHCl3 + alc.KOH →
(ii) C6H5N2Cl + H3PO2 + H2O →
(iii) ( ) C6H5NH2 + H2SO4 conc. →
(iv) C6H5N2Cl + C2H5OH →
(v) ( ) C6H5NH2 +Br2 aq →
(vi) ( ) 6 5 2 3 2 C H NH + CH CO O →
(vii) ( ) ( ) 4 2 i HBF 6 5 2 ii NaNO /Cu, C H N Cl Δ→
Question 13.12 Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Question 13.13 Write the reactions of (i) aromatic and (ii) aliphatic primary amines with nitrous acid.
Question 13.14 Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable
molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
:: Chapter 14 - Biomolecules ::
Question 14.1 What are monosaccharides?
Question 14.2 What are reducing sugars?
Question 14.3 Write two main functions of carbohydrates in plants.
Question 14.4 Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Question 14.5 What do you understand by the term glycosidic linkage?
Question 14.6 What is glycogen? How is it different from starch?
Question 14.7 What are the hydrolysis products of (i) sucrose and (ii) lactose?
Question 14.8 What is the basic structural difference between starch and cellulose?
Question 14.9 What happens when D-glucose is treated with the following reagents?
(i) HI
(ii) Bromine water
(iii) HNO3
Question 14.10 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Question 14.11 What are essential and non-essential amino acids? Give two examples of each type.
Question 14.12 Define the following as related to proteins
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Question 14.13 What are the common types of secondary structure of proteins?
Question 14.14 What type of bonding helps in stabilising the α-helix structure of proteins?
Question 14.15 Differentiate between globular and fibrous proteins.
Question 14.16 How do you explain the amphoteric behaviour of amino acids?
Question 14.17 What are enzymes?
Question 14.18 What is the effect of denaturation on the structure of proteins?
Question 14.19 How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Question 14.20 Why are vitamin A and vitamin C essential to us? Give their important sources.
Question 14.21 What are nucleic acids? Mention their two important functions.
Question 14.22 What is the difference between a nucleoside and a nucleotide?
Question 14.23 The two strands in DNA are not identical but are complementary. Explain.
Question 14.24 Write the important structural and functional differences between DNA and RNA.
Question 14.25 What are the different types of RNA found in the cell?
:: Chapter 15 - Polymers ::
Question 15.1 Explain the terms polymer and monomer.
Question 15.2 What are natural and synthetic polymers? Give two examples of each type.
Question 15.3 Distinguish between the terms homopolymer and copolymer and give an example of each.
Question 15.4 How do you explain the functionality of a monomer?
Question 15.5 Define the term polymerisation.
Question 15.6 Is ( NH-CHR-CO )n, a homopolymer or copolymer?
Question 15.7 In which classes, the polymers are classified on the basis of molecular forces? Question 15.8 How can you differentiate between addition and condensation polymerisation?
Question 15.9 Explain the term copolymerisation and give two examples.
Question 15.10 Write the free radical mechanism for the polymerisation of ethene.
Question 15.11 Define thermoplastics and thermosetting polymers with two examples of each.
Question 15.12 Write the monomers used for getting the following polymers.
(i) Polyvinyl chloride
(ii) Teflon
(iii) Bakelite
Question 15.13 Write the name and structure of one of the common initiators used in free radical addition polymerisation.
Question 15.14 How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Question 15.15 Discuss the main purpose of vulcanisation of rubber.
Question 15.16 What are the monomeric repeating units of Nylon-6 and Nylon-6,6?
Question 15.17 Write the names and structures of the monomers of the following polymers:
(i) Buna-S
(ii) Buna-N
(iii) Dacron
(iv) Neoprene
Question 15.18 Identify the monomer in the following polymeric structures.
Question 15.19 How is dacron obtained from ethylene glycol and terephthalic acid ?
Question 15.20 What is a biodegradable polymer ? Give an example of a biodegradable aliphatic polyester.
:: Chapter 16 - Chemistry in Everyday Life ::
Question 16.1 Why do we need to classify drugs in different ways ?
Question 16.2 Explain the term, target molecules or drug targets as used in medicinal chemistry.
Question 16.3 Name the macromolecules that are chosen as drug targets.
Question 16.4 Why should not medicines be taken without consulting doctors ?
Question 16.5 Define the term chemotherapy.
Question 16.6 Which forces are involved in holding the drugs to the active site of enzymes ?
Question 16.7 While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other ?
Question 16.8 Low level of noradrenaline is the cause of depression. What type of drugs are needed to cure this problem ? Name two drugs.
Question 16.9 What is meant by the term ‘broad spectrum antibiotics’ ? Explain.
Question 16.10 How do antiseptics differ from disinfectants ? Give one example of each.
Question 16.11 Why are cimetidine and ranitidine better antacids than sodium hydrogencarbonate or magnesium or aluminium hydroxide ?
Question 16.12 Name a substance which can be used as an antiseptic as well as disinfectant.\
Question 16.13 What are the main constituents of dettol ?
Question 16.14 What is tincture of iodine ? What is its use ?
Question 16.15 What are food preservatives ?
Question 16.16 Why is use of aspartame limited to cold foods and drinks ?
Question 16.17 What are artificial sweetening agents ? Give two examples.
Question 16.18 Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Question 16.19 What problem arises in using alitame as artificial sweetener ?
Question 16.20 How are synthetic detergents better than soaps
?
Question 16.21 Explain the following terms with suitable examples
(i) cationic detergents
(ii) anionic detergents
(iii) non-ionic detergents.
Question 16.22 What are biodegradable and non-biodegradable detergents ? Give one example of each.
Question 16.23 Why do soaps not work in hard water ?
Question 16.24 Can you use soaps and synthetic detergents to check the hardness of water ?
Question 16.25 Explain the cleansing action of soaps.

